Developing Diagnostic Tests for Canine Distemper Exposure
28/07/2025
Read more about the background to this project here.
University of Kent Project Report:
Project report for end of phase 2
This collaborative project between bioscientists at the University of Kent and conservationists from the University of Kent and Cornell University, aims to develop rapid diagnostics for the determination of the exposure of wild big cats, and other species, to Canine Distemper Virus (CDV) in a field-based setting. The work has been partially funded by Wildlife Vets International (WVI), whose support has enabled two MSc by research students (Grace Lelliott and Charlotte Dalzell) to work towards the goals of the project and obtain state-of-the-art training in the areas of biotechnology and diagnostics development.
The work described here follows on from the successful outputs of the first phase of the project. These were;
1. the development of a flexible bacterial expression plasmid for the production of viral proteins, and
2. the design, synthesis and cloning of genetic information to produce CDV DNA sequences for the production and analysis of the key protein antigen sequences required for the subsequent development of a diagnostic device.
This work enabled Grace and Charlotte to produce fragments of the CDV H protein, observed on the surface of the virus particle, and considered an excellent target for diagnostic development.
A key requirement of producing a species non-specific diagnostic is the ability to detect anti-CDV antibodies raised in response to infection across different species. Protein A/G bacterial proteins known to bind to antibodies from different species, are a potential solution to this. The utilisation of Protein A/G in conjunction with anti-CDV antibodies was the focus of the second phase of the project.
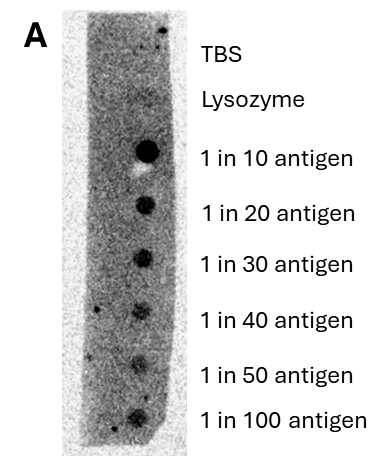
To determine the potential of the CDV H protein fragments to bind antibodies and be detected by protein A/G, a method of immobilisation was required. Initial efforts at assessing this focussed on the coating of 96 well plates with the protein fragments. This is a key component of Enzyme Linked Immunosorbent Assays (ELISA) used in diagnostic tools. To minimise cost, initial efforts to show successful coating of the plate and the ability of the immobilised protein antigen to bind to an antibody utilised a non CDV anti-his tag antibody which targeted the His-tag sequence engineered into the H fragment proteins produced. antibodies. Previous work under phase 1 had highlighted the potential of this his-tag antibody to detect the protein fragments of interest. This panel of experiments highlighted significant issues associated with the process of immobilising CDV proteins onto the plastic of the 96 well plate, which meant subsequent detection was difficult. As one of the end points of the project is the development of a lateral flow device (LFD) for the detection of anti-CDV antibodies, it was decided to circumvent the ELISA immobilisation issue by immobilising the recombinant H protein fragments onto a nitrocellulose membrane as used in LFDs.
Using a dot blot approach, whereby dilutions of the protein were dotted on to the membrane and then probed/visualise (see Fig. 1), it was pleasing to see that the recombinant protein did indeed adhere to the membrane and was still able to be bound, and detected, by an anti-His tag antibody, CDV antibody and a serum sample containing CDV antibodies (see Fig. 2).
This is an exciting step towards the development of a rapid diagnostic for field testing to detect previous exposure of animals, such as big cats, to CDV. The ability to detect such animals will provide knowledge that will enable conservation decisions to be made that will protect these already endangered species.

We would like to thank the Big Give Christmas Challenge funds and WildCats Conservation Alliance for enabling the development of this tool, which will be ground breaking.
.png)
.png)

.png)
.png)







.png)






.png)
.png)




.png)


